X Chromosome Inactivation: A Breakthrough in Genetic Therapy
X chromosome inactivation is a fascinating biological process that plays a crucial role in genetic regulation within female cells. In females, the presence of two X chromosomes creates an intriguing dilemma, leading to the silencing of one to prevent an overexpression of X-linked genes. This unique mechanism of chromosomal silencing is essential in preventing genetic disorders such as Fragile X Syndrome and Rett Syndrome, which arise from mutations on the X chromosome. Recent discoveries by researchers, including the influential work of Jeannie Lee, have highlighted the important role of the Xist molecule, which orchestrates this inactivation. By understanding how X chromosome inactivation works, scientists hope to unlock new treatments for conditions linked to genetic abnormalities, providing hope to those affected by these disorders.
The process of X chromosome inactivation, often abbreviated as XCI, involves a complex network of interactions that lead to the selective silencing of one X chromosome in females. This phenomenon is a critical aspect of dosage compensation in mammals, ensuring that both male and female cells express X-linked genes at similar levels. Researchers refer to this process using various terms including chromosomal silencing and gene regulation, emphasizing its significance in combatting genetic conditions such as Fragile X and Rett syndromes. The pivotal role of the Xist RNA molecule in mediating this inactivation highlights the intricate mechanics of cellular processes aimed at maintaining genetic balance. As scientists delve deeper into chromosomal dynamics, they uncover potential therapeutic avenues that may render previously hidden genes active, transforming the landscape of genetic disorder treatment.
Understanding X Chromosome Inactivation
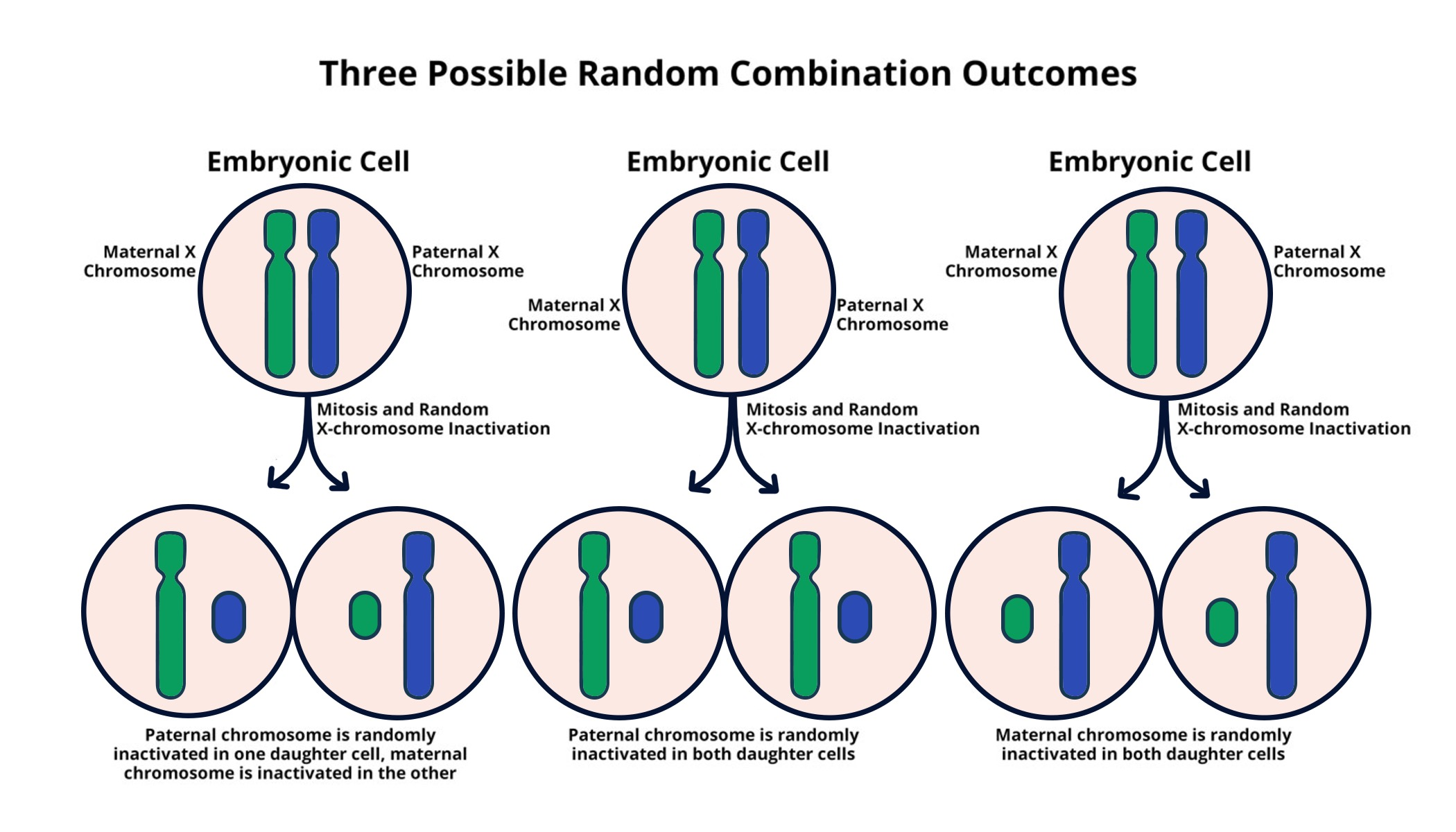
X chromosome inactivation (XCI) is a critical process that ensures dosage compensation between males and females. In females, having two X chromosomes is not necessary for proper gene expression since males possess only one. Therefore, one X chromosome in females is inactivated, effectively silencing its gene expression. This phenomenon has profound implications in genetics, particularly concerning disorders linked to the X chromosome. Researchers like Jeannie T. Lee and her team at Massachusetts General Hospital have dedicated decades to dissecting the intricacies of XCI, shedding light on mechanisms that govern chromosomal silencing and gene regulation.
The inactivation process involves complex interactions at the molecular level, prominently featuring the Xist RNA molecule. Xist plays an indispensable role in mediating the silencing of one X chromosome by changing the structural properties of chromatin, which is a fundamental aspect of how gene expression is regulated. As the Xist RNA coats the inactivated X chromosome, it alters the biophysical environment of the surrounding ‘Jell-O-like’ substance, facilitating a unique compartmentalization that allows for a controlled shutdown of gene activity. This process of X chromosome inactivation is not only crucial for normal development but also serves as a basis for understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome.
The Role of Xist in Genetic Disorders
Xist, or X-inactive specific transcript, is a powerful molecule that orchestrates the silencing of one of the X chromosomes in female cells. Its primary function revolves around chromosomal organization and gene regulation, making it an essential factor for preventing the overexpression of X-linked genes. The misregulation of Xist can lead to various genetic disorders, highlighting its importance in maintaining chromosomal integrity. Fragile X Syndrome, characterized by intellectual disability, and Rett Syndrome, associated with neurodevelopmental irregularities, both have links to the failure of appropriate X chromosome inactivation, resulting in aberrant gene expression.
Research into the mechanisms by which Xist functions has opened new avenues for therapeutic development. By understanding how Xist interacts with chromatin and its ‘Jell-O-like’ surroundings, scientists hope to develop innovative strategies to unsilence genes that are otherwise rendered inactive. This approach has the potential to not only ameliorate symptoms of X-linked genetic disorders but also provide insights into broader applications for chromosomal therapies. The implications of optimizing XCI techniques could lead to breakthroughs in gene therapy, catering specifically to patients with mutations that disrupt normal X chromosome functionality.
Potential Therapeutic Applications of XCI Research
The implications of research on X chromosome inactivation extend far beyond basic cell biology; they hold promise for therapeutic interventions in genetic disorders. For instance, Jeannie Lee’s group is actively exploring the use of Xist-based therapies that can activate silenced genes in individuals affected by Fragile X Syndrome and Rett Syndrome. By manipulating the inactivation process, researchers aim to salvage the functional resources of healthy genes that reside within the inactivated X chromosome. This approach could revolutionize treatment paradigms for conditions that are currently difficult to manage, providing hope to thousands affected by these disorders.
Several promising strategies are being developed to leverage Xist’s potent gene silencing capabilities. By tailoring compounds that can modify the chromatin landscape surrounding the X chromosome, scientists are endeavouring to generate treatments that unmask the latent potential of healthy genes in patients. Initial studies suggest that restoring functionality to inactivated X-linked genes could provide significant benefits with minimal side effects. This underscores the necessity for thorough safety testing and optimization of these therapeutic candidates before they can be progressed to clinical trials, paving the way for innovative interventions that could change the lives of those suffering from X-linked disorders.
The Mechanisms Behind Chromosomal Silencing
Chromosomal silencing is a complex and critical process that allows cells to appropriately regulate gene expression, particularly in the context of X chromosome inactivation. This mechanism is crucial for maintaining genetic balance, especially in females who possess two X chromosomes. As cells initiate XCI, a cascade of molecular events underpins the silencing process. At the core of this orchestrated effort is Xist, which coats the X chromosome and recruits various chromatin-modifying factors that facilitate the transformation of chromatin into a silenced state. This biophysical modification ensures that the inactivated X remains transcriptionally dormant.
Recent studies reveal that the dynamic properties of the chromatin structure are altered significantly due to Xist binding. This alteration creates a more pliable and accessible environment, allowing silencing factors to infiltrate deeply, impacting gene expression patterns remarkably. The understanding of this chromosomal silencing has implications not just for X-linked disorders but also for developing broader therapeutic strategies that target gene regulation mechanisms across various genetic disorders. The advancements made in this field could provide powerful insights into manipulating gene expression and correcting pathogenic gene activity.
Linking XCI to Genetic Disorders
The connection between X chromosome inactivation and genetic disorders has garnered significant attention from scientists exploring the etiology of diseases such as Fragile X Syndrome and Rett Syndrome. These conditions are primarily associated with mutations on the X chromosome and are exacerbated by the complexities of gene inactivation. Understanding how XCI operates is crucial for devising effective treatments, especially since many affected individuals carry mutations on only one X chromosome. Therapeutic strategies that focus on releasing gene silencing could utilize the intact alleles residing on the inactivated chromosome, paving the way for innovative care options.
As research progresses, the implications of linking chromosomal mechanisms with genetic disorders become increasingly evident. The ability to manipulate XCI not only holds promise for treating conditions like Fragile X Syndrome and Rett Syndrome but also raises questions about the broader impact of gene expression regulation. Genetic therapies that seek to modify inactivation processes may one day offer compelling avenues for restoring healthy gene function in patients with a range of genetic conditions, ultimately transforming approaches to genetic healthcare.
Challenges in X Chromosome Research
Despite the significant advancements made in understanding X chromosome inactivation, substantial challenges persist in the field of genetic research. Factors governing XCI are intricate, involving a variety of molecular players, including Xist and numerous chromatin remodelers. The complexity of interactions raises questions regarding how these processes can be effectively targeted in a therapeutic context. Researchers are continuously investigating the various pathways and mechanisms that contribute to XCI with hopes of translating this knowledge into viable treatments for conditions tied to X chromosome dysfunction.
Moreover, the ability to restore functionality to inactivated X-linked genes poses its own set of challenges. The restoration must be precise, ensuring that therapeutic interventions do not inadvertently affect other genes on the X chromosome that are crucial for normal cellular function. Research teams are working diligently to refine their understanding of gene regulation, aiming to devise solutions that minimize risks while maximizing therapeutic benefits for individuals suffering from genetic disorders. Addressing these challenges will be paramount for the successful application of new treatments designed to target X-linked genetic conditions.
Future Directions in Chromosomal Therapy
Looking forward, the development of effective chromosomal therapies based on X chromosome inactivation research is ripe with potential. Scientists are exploring various therapeutic modalities, including the use of RNA-based strategies targeting the Xist molecule to modulate gene expression patterns. As knowledge of the molecular underpinnings of XCI expands, novel approaches may emerge that empower clinicians to activate silenced genes in patients with X-linked disorders. The promise of targeted therapies provides hope for transforming the lives of individuals grappling with conditions like Fragile X Syndrome and Rett Syndrome.
The future of chromosomal therapy also hinges on interdisciplinary collaboration among geneticists, molecular biologists, and clinical practitioners. By merging insights from different fields, researchers will likely accelerate the pace of discovery, ultimately bringing therapeutic options to the forefront of medical practice. As the complexities of XCI become clearer, potential breakthroughs in treatment accessibility and efficacy will enable better outcomes for patients, demonstrating the impactful intersection of basic research and clinical innovation.
The Significance of Jell-O-like Substance in Chromosomal Function
The Jell-O-like substance surrounding chromosomes plays an important role in maintaining genetic organization and function. This gelatinous material provides a structural framework that prevents entanglement of chromosomes during cellular division, essentially functioning as a cellular lubricant. In the context of X chromosome inactivation, this substance facilitates the recruitment of Xist and other silencing factors, enabling effective chromosomal silencing. The interplay between Xist and this Jell-O-like substance is critical for the regulation of gene activity and the maintenance of chromosomal integrity.
Understanding the properties of this Jell-O-like substance can illuminate pathways through which chromosomal organization impacts gene expression. As researchers continue to study its biophysical properties, they may uncover innovative methods to refine therapeutic strategies targeting X-linked genetic disorders. By leveraging the unique characteristics of this chromatin-associated material, future therapies may mimic or enhance the silencing effects of Xist, leading to targeted treatments that restore healthful gene expression patterns in affected individuals.
Implications of Chromosomal Studies for Broader Genetic Research
The research surrounding X chromosome inactivation not only holds implications for specific disorders like Fragile X Syndrome and Rett Syndrome but also provides a framework for understanding genetic regulation across the genome. Insights gained from studies on XCI are informing broader concepts of chromosomal dynamics, gene expression, and epigenetic regulation. As scientists delve deeper into these processes, they are likely to uncover universal principles that govern gene interaction across sex chromosomes and autosomes alike.
The significance of understanding chromosomal behaviors extends into therapeutic development, as researchers explore how these principles can be harnessed for precision medicine. By incorporating lessons learned from XCI research into broader contexts, scientists may develop targeted therapies that address a wider array of genetic conditions. This cross-pollination of ideas will undoubtedly enhance the efficacy of future genetic interventions, demonstrating the interconnectedness of chromosomal biology and human health.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic disorders?
X chromosome inactivation (XCI) is a crucial biological process that occurs in females, where one of the two X chromosomes is randomly selected for silencing. This process is vital for maintaining gene dosage balance between males (who have one X chromosome) and females. Genetic disorders such as Fragile X Syndrome and Rett Syndrome are often associated with mutations on the X chromosome, making understanding XCI important for developing potential treatments.
How does the Xist molecule contribute to X chromosome inactivation?
The Xist molecule plays a pivotal role in X chromosome inactivation (XCI). It is an RNA molecule that coats the X chromosome designated for silencing, influencing the physical properties of the chromosomal environment. By modifying the surrounding material, Xist facilitates the chromosome’s inactivation, thus rendering genes on that X chromosome inactive, which is particularly relevant for conditions like Fragile X Syndrome and Rett Syndrome.
Can X chromosome inactivation be reversed to treat Fragile X Syndrome and Rett Syndrome?
Recent research suggests that reversing X chromosome inactivation could be a therapeutic approach for treating diseases like Fragile X Syndrome and Rett Syndrome. By unsilencing the mutated genes on the inactivated X chromosome, researchers aim to restore gene function. This exciting potential opens doors for new treatments that target the underlying genetic basis of these disorders.
What role does chromosomal silencing play in sex differences and genetic disorders?
Chromosomal silencing, specifically X chromosome inactivation, is a critical process that ensures gene dosage balance between sexes. For females, having two X chromosomes could lead to an excess of X-linked gene products if both were active. In genetic disorders, improper inactivation might exacerbate conditions like Fragile X Syndrome or Rett Syndrome, highlighting the significance of understanding XCI in both basic biology and disease treatment.
How might understanding X chromosome inactivation lead to breakthroughs in therapy for genetic disorders?
Understanding X chromosome inactivation could lead to therapeutic breakthroughs by enabling scientists to manipulate the process for beneficial outcomes. For instance, the ability to unsilence genes on the inactivated X chromosome may help restore function to genes affected by mutations found in disorders like Fragile X Syndrome and Rett Syndrome, creating new avenues for effective treatment.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes while males have one. To compensate, one X is inactivated in females. |
| Role of Xist | A gene on the X chromosome produces an RNA molecule called Xist, which alters the surrounding chromatin structure to lead to inactivation. |
| Biophysical Changes | Xist interacts with a gelatinous substance around the chromosome, making it more flexible and allowing for successful inactivation. |
| Implications for Genetic Disorders | Potential treatments for Fragile X Syndrome and Rett Syndrome by reversing X-inactivation to access healthy genes. |
| Current Research Focus | Safety studies and optimization of therapies over the next few years with plans for clinical trials. |
| Unanswered Questions | Understanding why certain healthy genes remain unaffected when mutated genes are restored. |
Summary
X chromosome inactivation is a crucial process in females that allows one of the two X chromosomes to be silenced, ensuring that the gene dosage is balanced with males who have only one X. The groundbreaking research by Jeannie T. Lee’s team explores how this inactivation occurs, revealing the significant role of the Xist RNA molecule in modifying the chromatin environment around the chromosome. This insight paves the way for therapeutic potential in treating X-related genetic disorders, such as Fragile X and Rett syndromes. As the research progresses toward clinical application, it highlights the importance of understanding X chromosome inactivation not just for basic biology, but for developing effective treatments for debilitating genetic conditions.